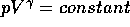
The launch, itself, can be assumed to be an adiabatic process,
where
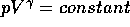
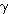 =1.4.
The work done by the gas during adiabatic expansion is given by
=1.4.
The work done by the gas during adiabatic expansion is given by
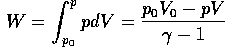
According to the gas law, pV/T=constant, and this expression can be used to relate the temperature during the adiabatic expansion to the pressure or the volume.
The relations above can be expressed in terms of dimensionless
quantities depending on the ratio V/Vo. The maximum possible
work for an adiabaticlally expanding gas
would be
Wo=poVo/( See also a comparison of data
for launch coasters.
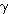 -1).
which depends on the initial pressure and volume
-1).
which depends on the initial pressure and volume
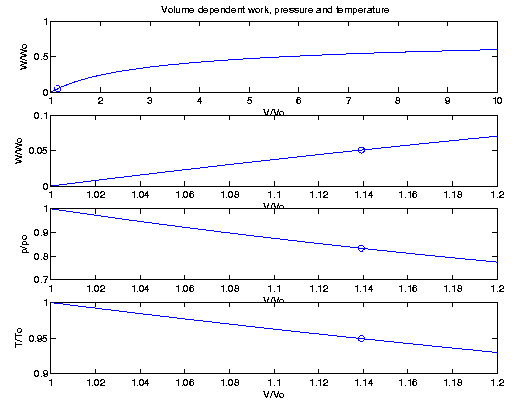
Figure 1.
http://fy.chalmers.se/LISEBERG/KANONEN/launch.html
2005-07-25 AMP